Chemical Reactions of Aldehydes and Ketones
Chemical Reactions of Aldehydes and Ketones: Overview
This topic covers concepts, such as, Chemical Properties of Aldehydes and Ketones, Nucleophilic Addition Reactions of Aldehyde and Ketones, Reaction of Aldehydes and Ketones with Fehling's Reagent & Haloform Reaction etc.
Important Questions on Chemical Reactions of Aldehydes and Ketones
How does sulphonation reaction take place?
The nitration in aryl aldehyde produces meta products-justify.
Formaldehyde and benzaldehyde can be involved in aldol condensation.
When carbonyl compounds react with hydroxylamine produces _____.
Give the IUPAC name of the only aldehyde which undergo iodoform test.
Which of the following will neither undergo an aldol condensation reaction nor a Cannizzaro reaction?
Which of the following reaction, will not give a yellow precipitate?
The order of susceptibility of nucleophilic attack on aldehydes follows the order
In Clemmensen Reduction carbonyl compound is treated with _____.
Which of the following is the disproportionation redox reaction?
Aldol condensation reaction is not given by
Clemmensen reduction
Which of the following properties of the carbonyl group allows aldehydes and ketones to undergo nucleophilic addition reactions?
Which of the following method is used for conversion of the ketone to hydrocarbon?
Compounds and in the following reaction are _____.
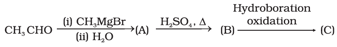
Which of the following method is used for conversion of ketone to hydrocarbon?
When ethanal is heated with Fehling's solution, then the precipitate obtained is of:
Aldehydes and ketones do not react with :
The reduction of acetone by gives:
Which of the following is used for reduction of aldehydes and ketones in Clemmenson's reduction ?
